
23rd June 2014 – Organic catalysts are essential for a number of industrial applications, but their inability to work within the same system or in water means that their efficiency is somewhat limited. Researchers from the Eindhoven University of Technology believe that they may have solved this problem by taking a leaf out of the structure of nature’s own catalysts – enzymes. This could help to make industrial processes such as drug manufacturing both faster and cheaper.
Catalysts used in organic chemistry, on the other hand, are quite different to enzymes. They are typically much smaller molecules that do not have large three-dimensional structures around them, and thus tend to be much less selective. However, it is often the case that these catalysts can stimulate reactions that enzymes cannot.
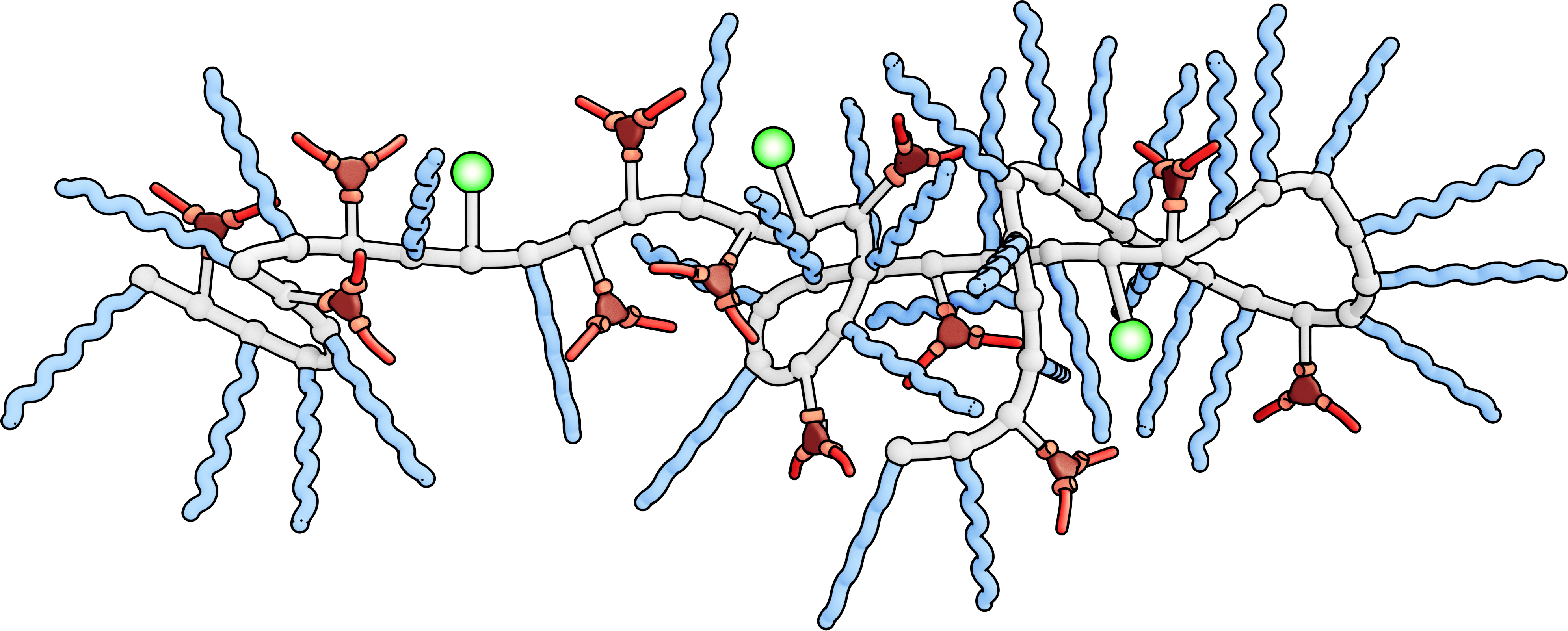
Is there a way to get the best of both worlds? Dr Anja Palmans of the Eindhoven University of Technology thinks so. “We can mimic the three-dimensional structure of an enzyme using polymer chains,” she explains. “Using what is known as a supramolecular recognition unit, we can fold these chains into compartmentalised architectures much similar to enzymes, which we can then insert a catalytic core into. The folded polymer chain will have a hydrophilic outer surface similar to an enzyme, allowing the synthetic catalyst to work in water.”
The possibilities opened up by this research are numerous. Enzyme-like activity in a completely synthetic system could be used for reaction cascades in which multiple reactions are occurring at once in the same environment. “When making drugs, for example, the current process involves carrying out one reaction, isolating the product and then purifying the product before moving on to the next reaction and repeating the whole process,” explains Palmans. “This is because standard organic catalysts tend to inhibit or alter each other’s activity and so cannot be used within the same system.”
“However, with these synthetic catalysts the active site is shielded and so they do not interfere with each other. This allows one to have a system in which a number of reactions can be happening simultaneously within a single procedure.”
The Eindhoven University of Technology has been the base of this research, providing a unique multidisciplinary environment that has been fundamental to the success of the work, according to Palmans. “The institute we work in, the Institute for Complex Molecular Systems, was specifically created so that researchers from a number of different disciplines can work within the same building,” she says. “We have polymer chemists to work with the polymer chains, organic chemists to develop the catalysts and supramolecular recognition units, polymer physicists to aid our understanding of the folding and to bring complex methods of analysis, as well as mathematicians who utilise their knowledge of modelling.”
The first experiments on cascade catalytic systems are now running, but the next step will be to relate the structure of the polymers back to the catalytic activity. “We can now shield the catalysts within the polymer; that has been proven,” says Palmans, “but what we really want to do now is to get back to design principals so that we can improve the levels of catalysis. The mathematicians we are working with will be crucial for this step, as their knowledge of models will allow us to work on molecular design.”
It will be another few years before the final results of this intriguing research are published, and it could be that it helps to revolutionise the use of organic catalysts.
